AIM Applies for Approval to Manufacture and Market the World's First Gastric Cancer AI
AI MEDICAL SERVICE INC., hereinafter AIM, whose head office is at Toshima-ku, Tokyo, and whose CEO is Tomohiro Tada, has applied to the Minister of Health, Labour and Welfare for a medical device manufacturing and marketing approval for its AI-based endoscopic diagnosis support system. The endoscopic AI judges the neoplastic or non-neoplastic nature of gastric lesions, hereinafter referred to as "gastric cancer differentiation AI", based on the "Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices.” If approved, AIM’s product will be the world's first (*1) AI-based endoscopic diagnosis support system related to stomach disease.
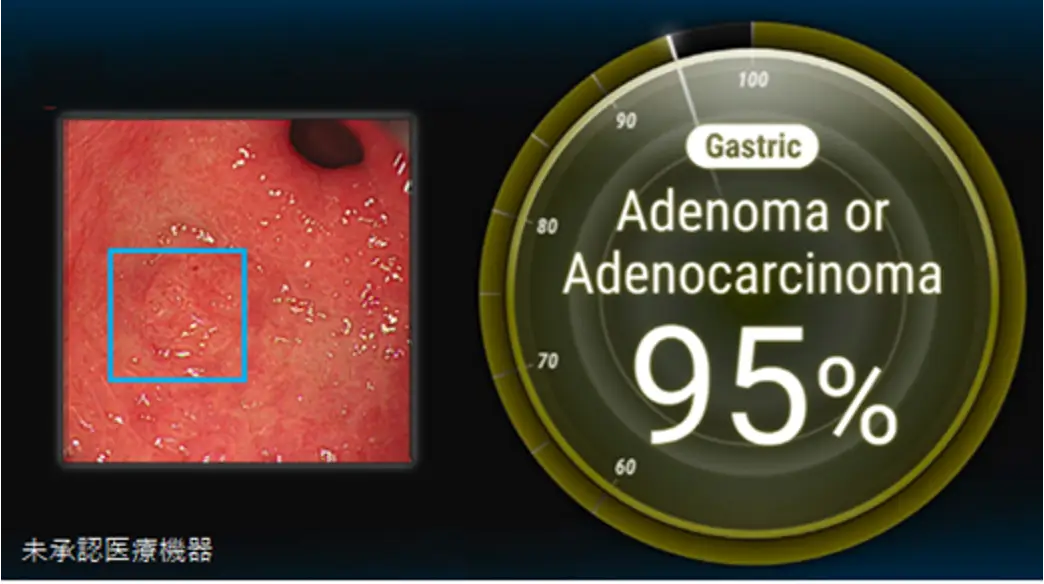
■ Background of Development
In Japan, the number of new gastric cancer cases each year is second only to colorectal cancer, with more than 120,000 cases each year; the number of deaths is the third most after lung cancer and colorectal cancer, with more than 40,000 deaths each year (*2). Gastric cancer is characterized by an increasing mortality rate with disease progression. However, gastric cancer is very often treatable if detected at an early stage; the 5-year relative survival rate is less than 50% if detected at stage III or later, but more than 95% if detected at stage I. On the other hand, early-stage gastric cancer is difficult to detect and is said to be missed in 4.5-25.8% of cases.
Against this backdrop, AIM developed an endoscopic AI specialized in gastric cancer. The use of this AI will enable non-specialists to detect early gastric cancer with the same level of accuracy as specialists. Use of the endoscopic AI is expected to reduce the number of missed early-stage gastric cancers and standardize the quality of endoscopic examinations.
The use of the gastric cancer AI presents a win-win solution for both patients and physicians. For the patient, the endoscopic exam is conducted as normal, but the endoscopic AI allows for more accurate diagnoses. For physicians, employing the AI with minimal additional training or workflow burdens can reduce the psychological burden of diagnostic decision-making. Therefore, AIM believes that the barriers to adopting the gastric cancer AI are relatively low such that it will eventually become widely accepted.
■ Future Development
With the mission of "saving patients around the world," we would like to introduce endoscopic AI, including the gastric cancer AI, to clinical practice not only in Japan but also around the world. Japanese manufacturers lead the world in endoscopic system development and sales, and Japanese institutes have accumulated the highest quality and largest volume of medical imaging data. In April 2021, AIM established a joint research agreement with the National University Hospital of Singapore, one of the top universities in Asia (*3). By developing an endoscopic AI that draws on the wisdom of Japanese endoscopists, our company hopes to improve endoscopic care around the world and save as many patients as possible.
*1: According to our own research
*2:Latest cancer statistics https://ganjoho.jp/reg_stat/statistics/stat/summary.html [Accessed 2021/08/31]
*3:Click here for the notice of this matter: https://www.ai-ms.com/20210428/473/
For inquiries, please contact
AI Medical Service Inc.
Hareza Tower 11F, 1-18-1 Higashi-Ikebukuro, Toshima-ku, Tokyo 170-0013, Japan
E-mail: Media, etc.: pr@ai-ms.com, Medical institutions: ask@ai-ms.com
Contact
Feel free to contact us using the form below regarding joint research,
media coverage, business partnerships, or related opportunities.