AI Medical Service Inc. Announces Regulatory Approval of Gastric AI-based Endoscopic Imaging Diagnostic Support System
- Real-time double-checking of areas suspected of early gastric neoplasia -
AI MEDICAL SERVICE INC. (hereinafter AIM), a medical start-up specializing in the development of diagnostic endoscopic AI, is excited to announce the manufacturing and marketing approval of “gastroAI™ model-G2,” an AI-based endoscopic detection support device that operates within the stomach. The regulatory approval was granted by Japan’s Minister of Health, Labour and Welfare (MHLW) on December 17, 2024. The endoscopic AI (hereinafter referred to as “Gastric Neoplasia Detection Support AI”) analyzes endoscopic images of the stomach captured by the endoscopic equipment during endoscopic examination. It then detects areas within the images suspicious for early gastric adenoma[1], and assists physicians in diagnosing those lesions. The product is scheduled to go on sale in the spring of 2025.

About gastric cancer detection support AI “gastroAI™ model-G2”
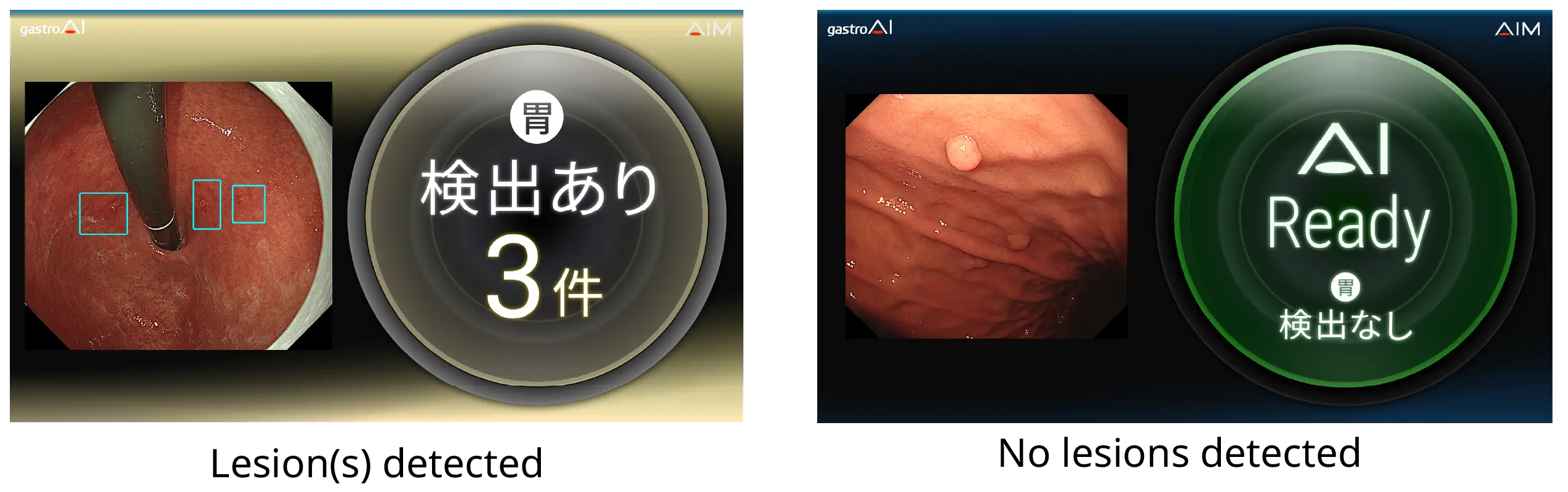
“gastroAI™ model-G2” analyzes images provided by endoscopic equipment, detects areas of suspected adenoma and early gastric cancer in the images, and helps the examiner identify these lesions by placing a frame within the image and playing a sound. These mechanisms help the observer interpret the image and detect lesions.[2]
Brand name | gastroAI |
Registration number | 30600BZX00266000 |
Main Features
1. Detection targets
The software’s detection target is an area or areas that are visually similar to cases of adenoma and early-stage gastric cancer.
2. Real-time double-checking during endoscopy
The lesion detection support function is initiated once the operator freezes the endoscopic image during the endoscopic exam, generating a still image that the AI analyzes before displaying the results of this analysis on the monitor. The AI analysis screen is displayed on a sub-monitor that stands independent of the main endoscopic system monitor. Therefore, use of the software does not interfere with the routine observation of endoscopic images. AI analysis takes less than 0.15 seconds to complete.
Performance Evaluation Test Results
| Sensitivity (A metric that measures the ability of a model to correctly identify positive cases.) | 91.4% |
| Specificity (A metric that measures the ability of a model to correctly identify negative cases.) | 90.3% |
1. Performance evaluation tests demonstrated improved sensitivity even when paired with experienced doctors[3].
AIM conducted a performance evaluation that compared the sensitivity and specificity of doctors in two cases: 1) using the product and, 2) not using the product. Even for experienced doctors, performance improved with use of the product.
2. Summary
Description: Retrospective reading test[4] with still images.
Subjects: Adenoma and early gastric cancer (raised, flat, or depressed) with suspected epithelial tumor.
Number of data: 150 images for positive cases (target lesion present), 350 images for negative cases (no target lesion present)
| Sensitivity | Specificity | |
| Experienced doctor | 66.4% | 90.8% |
| Experienced doctor + gastroAI™ | 83.5% | 92.9% |
Background of our efforts to obtain regulatory approval
In Japan, the number of new gastric cancer cases each year is third only to colorectal cancer and lung cancer, with more than 100,000 cases each year; the number of deaths is the third most after colorectal cancer and lung cancer, with more than 40,000 deaths each year.[5] Gastric cancer is characterized by a mortality rate that increases significantly with disease progression. Furthermore, gastric cancer is very often treatable if detected at an early stage; the 5-year relative survival rate is approximately 95% if detected at stage I, but less than 50% if detected at stage III or later. Nevertheless, early-stage gastric cancer is difficult to detect and is said to be missed (i.e., overlooked or misinterpreted) in 4.5 to 25.8% of cases.[6]
The newly approved “gastroAI™ model-G2” supports the examiner’s detection of lesions by analyzing endoscopic images and detecting areas within the image that are suspicious for adenoma or early gastric cancer. When used during endoscopic examination, gastroAI™ supports the detection of suspicious areas as if a skilled physician were double-checking those images in real time.
AIM will continue to strive to realize its mission of "Save Lives All Over the World" through endoscopic AI for diagnostic support.
Future Developments
With the mission of “Save Lives All Over the World,” AIM will deliver endoscopic AI (including but not limited to diagnostic support for gastric cancer) to clinical sites not only in Japan, but also around the world. Japanese manufacturers lead the world in the development and manufacturing of gastrointestinal endoscopes, and Japanese organizations have accumulated the highest quality and largest volume of imaging data. In the future, AIM will accelerate R&D efforts to expand the functions of AI for gastrointestinal diagnosis support, as well as to expand the target organs covered by this AI to solve further clinical problems.
By developing an endoscopic AI that draws on the wisdom and experience of Japanese endoscopists, we hope to improve endoscopic care in Japan and around the world to save as many patients as possible.
About AI Medical Service Inc.
AI Medical Service (AIM) is a Tokyo-based medtech company established with the mission to “Save Lives All Over the World.” Japan leads the world in endoscopic diagnosis and treatment, providing firms and researchers with access to large amounts of high-quality data. AIM is the leading player in the field of endoscopic AI, engaging in joint research with more than 140 medical institutions. By bringing endoscopic AI to the real-world clinical setting as soon as possible, AIM aims to reduce the number of missed cancer diagnoses and save lives around the world.
About CEO of AIM, Dr. Tomohiro Tada
Tomohiro TADA, M.D., Ph.D.
Dr. Tomohiro TADA is the CEO of AI Medical Service Inc., the chairperson of Tada Tomohiro The Institute of Gastroenterology & Proctology as well as Visiting Lecturer, Department of Surgical Oncology, Graduate School of Medicine, the University of Tokyo Hospital.
Dr. TADA received his M.D. of school of medicine in 1996 and Ph.D. of department of surgery in 2005 from the University of Tokyo. He trained in Colorectal Surgery at the University of Tokyo Hospital.
―――――――――
Company: AI Medical Service Inc.
Address: Hareza Tower 11F, 1-18-1 Higashiikebukuro, Toshima-ku, Tokyo 170-0013, Japan
CEO: Tomohiro Tada
Founding: September 1, 2017
Business: Development of Endoscopic AI
[1] Benign tumor. Large lesions may be treated endoscopically because they can become cancerous.
[2] The product is positioned as an “examiner’s aid in detecting lesions” and is not intended to support qualitative diagnosis or determine a treatment strategy based solely on the results of analysis using the product.
[3] Doctors who are board certified and have performed an average of 200 or more upper gastrointestinal endoscopies per year over last 5 years.
[4] Tests using anonymized medical data (removed medical records number, etc.) from past medical procedures.
[5] Reference: Cancer Today [Accessed 2024/12/17] https://gco.iarc.fr/today/
[6] Reference: Hosokawa et al. Hepatogastroenterology 2007 Mar; 54(74):442-4
Contact
Feel free to contact us using the form below regarding joint research,
media coverage, business partnerships, or related opportunities.